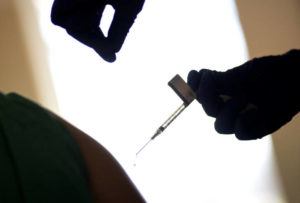
LONDON >> Britain on Wednesday approved the emergency use of a second COVID-19 vaccine, which has become the first country to become an easy-to-handle shot that developers hope will become the ‘vaccine for the world’.
The Department of Health said it had accepted a recommendation from the Regulatory Agency of Medicines and Healthcare Products Regulators to authorize the vaccine developed by the University of Oxford and the drug manufacturer AstraZeneca in the UK.
Britain has bought 100 million doses of the vaccine and plans to start injections within a few days. Hundreds of thousands of people in the UK have already received another vaccine, made by the American drug manufacturer Pfizer and the German firm BioNTech.
AstraZeneca CEO Pascal Soriot said it was an important day for millions of people in the UK to access this new vaccine. It has been shown to be effective, well tolerated, easily administered and delivered without any profit by AstraZeneca. ”
Partial results from studies in nearly 24,000 people in Britain, Brazil and South Africa indicate that the shots are safe and about 70% effective in preventing coronavirus infection.
It’s not as good as other vaccine candidates, but Soriot recently told the Sunday Times he is confident the vaccine will be just as effective as the competition.
Coronavirus vaccinations are usually given in two doses, with an initial shot followed by an enhancer about three weeks later.
But in a change of approach, the UK government has said that with the vaccination of AstraZeneca the priority will be given to giving as many people as possible a single dose, which is believed to provide a great deal of protection against the virus. It is said that people with the highest risk will get preference, and that everyone will get a second jerk within twelve weeks after the first.
The Oxford-AstraZeneca vaccine is expected to be relied on in many countries due to its low cost, availability and ease of use. It can be stored in refrigerators instead of the ultra-cold storage space that other vaccines need. The company said it would sell it for $ 2.50 per dose and plans to make up to 3 billion doses by the end of 2021.
“We have a vaccine for the world,” said one of the study leaders, Dr. Andrew Pollard, of Oxford said.
Researchers claim that the vaccine is protected against disease in 62% of those who receive two full doses, and in 90% of those who initially received half the dose due to a manufacturing defect. However, the second group included only 2,741 people – too few to be conclusive.
There are also questions about how well the vaccine protects older people. Only 12% of the study participants were older than 55 and they were enrolled later, so there was not enough time to see if they developed lower infections than those who did not receive the vaccine.
Researchers were also criticized in September for lack of information, when studies were stopped because a participant was suffering from a serious illness. AstraZeneca initially declined to provide further details due to patient confidentiality.
Eventually, the trials resumed after regulators checked safety data and decided it was safe to proceed. Published results show no hospitalizations or serious illnesses among those who received the vaccine.
The results announced so far are only partial as the studies continue. A separate study testing the AstraZeneca vaccine in the US is also underway.
The vaccine will now become the second COVID-19 vaccine to be used in Britain. On December 2, regulators gave similar emergency approval to the Pfizer-BioNTech vaccine.
Having another vaccine available means more people can get protection, says Sarah Gilbert, an Oxford scientist involved in the AstraZeneca project. It follows a different approach than the one or another Pfizer-BioNTech developed in the United States by Moderna Inc.
Gillies O’Bryan-Tear, chair of policy and communications at the British Faculty of Pharmaceutical Medicine, says the ultra-cold storage required by other vaccines is very impractical. This means that the AstraZeneca one “can reach more parts of the world than the Pfizer one,” he said.
Britain’s action is likely to mean that the World Health Organization will soon clear the AstraZeneca vaccine for use in a global effort to help poor countries, called COVAX. The initiative, led by WHO and the vaccine alliance GAVI, has ensured access to at least 100 million doses of the vaccine, with options and other offers to buy more. But no one can be distributed until it is lit by the WHO green.
The UN Health Agency does not license or regulate vaccines, but usually evaluates vaccines once they have been approved by an agency such as the UK regulator or the European Medicines Agency. WHO experts do their own evaluation of whether or not the risks of a vaccine outweigh its benefits, and then make a recommendation that the shots be ‘pre-qualified’ so that they can be purchased by developing country donors.
Most coronavirus vaccines used in poorer countries are likely to be made by the Serum Institute of India, which has been contracted by AstraZeneca to make 1 billion doses. In June, the pharmaceutical company announced that the Serum Institute would produce 400 million doses by the end of 2020, but as of early December, only about 50 million doses had been produced after production was stopped several times.