Getty Images
Lanzan alerts to women being vaccinated COVID Johnson & Johnson
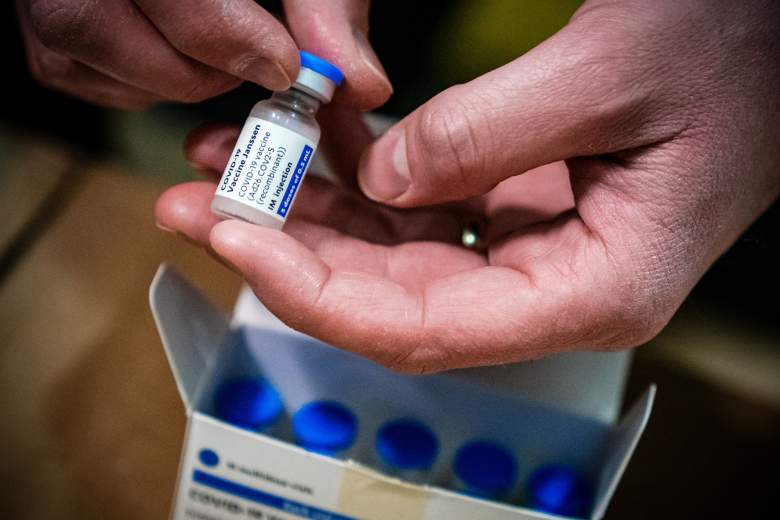
First of March, April 13, the CDC (Centers for Control and Prevention of Diseases) and the FDA (Food and Drug Administration of the United States) will suspend the distribution and administration of the vaccine against COVID-19 of a single dose manufactured by Johnson & Johnson and EStados Unidos.
The alert launched by the health authorities of the country, lies that it is known that 6 people have applied to report the disappearance of bullets, which is the cause of preoccupation.
The FDA has advised the FDA that it has now administered more than 6.8 million doses of the Johnson & Johnson vaccine in the country and is reviewing the dates of six cases reported of “a rare and serious blood clot in individuals having received the vaccine.
And has known the name of the federal bodies, which are in need of a detailed revision to the information of the registered coagulants, the question that many patients have applied to Johnson & Johnson’s vacancy is that they need to do it and they must be algal. type of preoccupation.
Even so, owning one is still beyond the reach of the average person.
The security of the vacancies for the # COVID-19 is a maximum priority for the federal governor, and we will take very seriously all the reports of past health problems in the evacuation against the COVID-19.
– US FDA and Español (@FDAenEspanol) 13 April 2021
“People who have received the J&J vaccine and who develop severe cerebral palsy, abdominal pain, pain in the paws or failure to breathe during the last three weeks of communication must be communicated with their medical attention provider”, manifest Peter Marks, Director of the FDA’s Evaluation and Biological Investigation Center and the Dra. Anne Schuchat, Deputy Director General of the CDC in a joint communication that can be completed here.
At the same time, staff officers will be called to the medical and clinical staff who are in contact with the vaccine and people vaccinated with the Johnson & Johnson dose.
“Apply for medical attention providers to notify adverts of the vacancy notification system in https://vaers.hhs.gov/reportevent.html”, aggregate.
However, the CDC and the FDA have been working on a series of allegations of coagulation, insisting that they should calm down.
The security of the vacancies for the # COVID-19 is a maximum priority for the federal governor, and we will take very seriously all the reports of past health problems in the evacuation against the COVID-19.
– US FDA and Español (@FDAenEspanol) 13 April 2021
“At the moment, these adversary events seem extremely rare. The safety of the COVID-19 vacancy is a maximum priority for the federal governor, and we will take seriously all the information on posterior health problems to the COVID-19 vacancy, ”officials said.

In these cases, a type of blood clot is observed called cerebral venous thrombosis (CVST) in combination with low blood platelet levels (thrombocytopenia). The six cases will occur between women from 18 to 48 years, and the cases will be presented from 6 to 13 days after the evacuation. The treatment of this specific type of blood clot is different from the treatment normally administered. Generally, an anticoagulant drug called heparin is used to treat blood clots. In this context, the administration of heparin can be dangerous and it is necessary to administer alternative treatments “, explains.
The FDA and the @CDCgov issue a Johnson & Johnson vacancy statement to the # COVID-19. We recommend a break in the use of this vacancy for precaution. https://t.co/EOaNEScaRL
– US FDA and Español (@FDAenEspanol) 13 April 2021
The CDC convenes a meeting of the Assessment Committee on Practice of Immunization (ACIP) to review more of these cases and evaluate their potential importance. The FDA is reviewing this analysis as well as investing in these cases. Once this process is complete, we recommend a break in the use of this vaccine by precaution, ”advises CDC and FDA officials. “It is important, on the one hand, to ensure that the Medical Attention Testers Association is aware of the potential of these adverse events and can plan the appropriate treatment and treatment required at the time required with this type of treatment.
Follow AhoraMismo on Instagram