Operation Warp Speed officials changed course on Tuesday over their deployments for vaccines, which expanded the recommendations for who should get COVID-19 shots and promised to send out more first doses, rather than discuss second shots.
The move, through the Trump administration’s $ 18 billion public-private partnership for vaccine development, comes amid a disappointingly slow first month of nationwide vaccine distribution. The change is aimed at maximizing the number of people receiving the first of their two shots, and providing some protection against a pandemic rising at unprecedented levels nationwide.
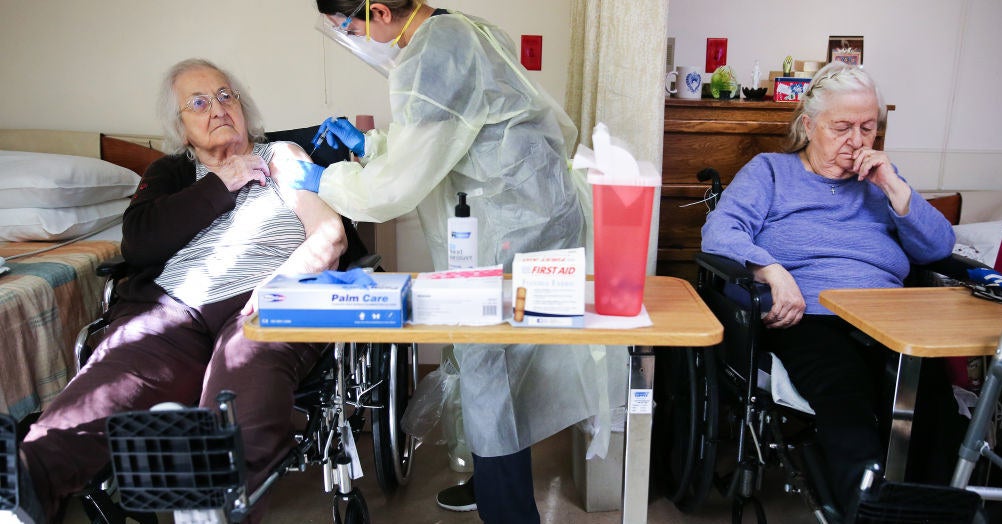
Vaccines will now be recommended for all people over the age of 65 or for anyone at high risk, such as diabetes, heart disease or obesity. The total population is about 90 million people.
The federal government will also assist states in setting up mass vaccination sites, Alex Azar, head of the department of health and health and human services, said Tuesday.
“We need doses to the place where it will be used the fastest,” Azar said. Allocations of vaccines starting in two weeks will be based on the size of the population of more than 65 years in each state, and they will have to report that they have used the vaccines, he added. “Going over to the wider population when the demand for vaccine supplies has always been part of the plan.”
In the past week alone, more than 1.7 million Americans have been diagnosed with COVID-19 and according to the CDC, 22,582 have died. The OWS changes come ahead of a Thursday briefing by Elected President Joe Biden, whose transition team last week announced similar plans to eject all available vaccine doses, as part of a promise of 100 million shots in the first 100 days of the Biden to give. administration.
After OWS initially had to distribute 300 million vaccine doses by January, OWS gradually reduced its projections to 20 million by December, a target it also missed, with less than 9 million people getting it for the first time on Monday.
The FDA has approved two vaccines, one made by Pfizer and the other by Moderna, for emergency use. Large-scale clinical trials have found that the vaccines are very protective against COVID-19 when given in two doses three or four weeks apart. But over the holidays, while facing a nationwide boom, driven mainly by a new extra-contagious coronavirus variant, the UK has extended the window for second-dose vaccines to as many as 12 weeks. Last week, an expert panel from the World Health Organization suggested that a six-week window be acceptable.
A tense public debate has since played out over the risks of continuing with only one chance to maximize the number of people who can be vaccinated in the US. Some fear that this could lead to people being partially immunized becoming infected and enabling the virus to develop resistance to the vaccines. Azar stressed on Tuesday that he believes producers will be able to produce enough doses to keep on schedule, and that it will still be a priority to get people their second survey.
Lost in the debate is that while some states like New York struggling with a lack of vaccine supply, many others had a lack of vaccinations to give the shots. Of more than 25 million shots fired by OWS at thousands of locations in 64 jurisdictions nationwide, only about a third of the shots were injected into humans.
In Los Angeles, where hospitals are being crushed by a record influx of seriously ill COVID-19 patients, Dodger Stadium, the largest test site in the country, is being transformed into a vaccination distribution center. California is currently the 46th nationwide in the number of doses it has administered per 100 people.
“I think this is the ultimate proof of problems with the U.S. health care system, which is fragmented, which probably has the wrong priorities,” Michigan University epidemiologist Arnold Monto, acting chairman of the FDA’s vaccination advisory committee, said during said an information session. held by the JAMA medical journal Monday. “We underestimated the challenges it would present, because we are really not as organized as health care countries as a more systematic part of government.”
Azar said Tuesday that such backlogs are being considered in which states are getting extra doses. “If you do not use the vaccines you have, we need to rebalance with the countries that are there,” Azar said. “Our states do not use 100% of their allocated vaccine.”
Part of the announcement includes an expansion of federal partnerships with pharmacies to deliver shots, Azar said. Hospitals are not where most people go to get vaccinated, and if they move to distribute shots to pharmacies, it will help reach more people in low-income and minority communities hardest hit by the pandemic, he said. suggested.