Getty Images
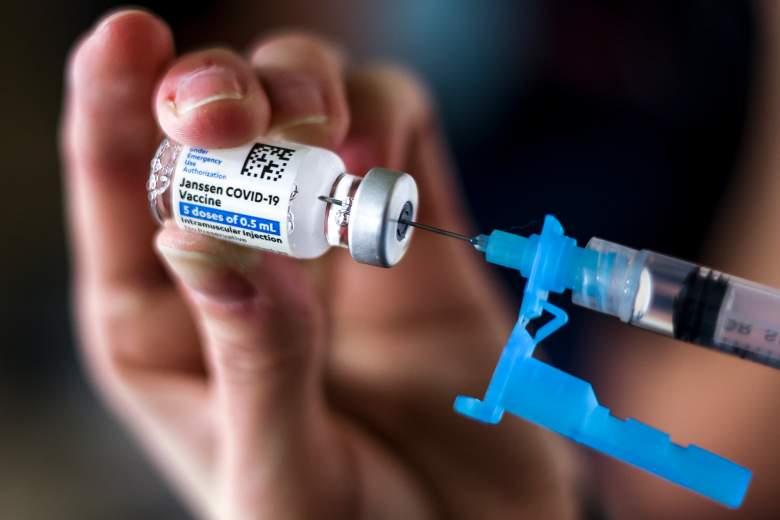
Tiffany Karschamroon extracts a dose of a Johnson & Johnson COVID-19 vial, approved by the EE FDA. UU.
According to information from the Reuters, EE.UU Food and Drug Administration. (FDA), the production of the Sars-Cov-2 vacuum contravirus from Johnson & Johnson, and a plant from the Emergent BioSolutions biopharmaceutical company, are currently investigating an error that occurred in March. Millions of pharmaceutical doses are lost.
The regulatory authorities regulate stadiums order the production of the vaccine against #Covid-19 de #Johnson& Johnson in a factory, where 15 million doses of the drug were presumably given. https://t.co/P5Ay26EQtm
– @ diario24horas (@ diario24horas) 20 April 2021
According to the Emergent BioSolutions agreement, the FDA authorities are launching a new inspection of its installations in Bayview, Baltimore, on April 12, and last Wednesday, the company’s request to “initiate the production of no new material in its Bayview installations and put in place the existing material manufactured in the Bayview installations has to be completed the inspection and repair of any resulting halo “, says the Washington Post periodical.
An error in the production of the vacuum
Detienen Johnson & Johnson’s production of an EE.UU plant. donde estropearon by error millions of pharmacy dose – RT https://t.co/CR8tAY3i9o
– Walter Goobar (@wgoobar) 20 April 2021
The crisis of a human error occurred on March 31, in a plant in Baltimore at a factory of the company Emergent BioSolutions, manufacturer of injectables by AstraZeneca and Johnson & Johnson. The fact that this production of this vaccine is present, radically changing plant plants, is accidentally combining the ingredients of medicinal plants for which as many as 15 million doses of Johnson & Johnson vaccine present manufacturing defects. Asimismo, it is established that the pregnancy is based on one of the ingredients used to elaborate the vaccine without passing the quality control.

The ingredients used in the manufacture of another vacuum cleaner, the AstraZeneca, were mixed with Johnson & Johnson vacuum cleaner, according to The New York Times Diary, quoted by Diario Las Americas.
This incident has been detrimental to Johnson & Johnson’s capacity in the case of a juvenile settlement, since only the vacancy rate has been announced, so that the authorities will close the factory as long as the investigations into the case are successful.
Signaling quality control detects the defective dose of Janssen’s vaccine, because these doses are only salaried from the production plant and, on the other hand, the production of AstraZeneca’s biological products is withdrawn from the plant.
“The human errors that occur”, the doctor Anthony Fauci, the epidemiologist of the Netherlands It is antidote to the preoccupation that has just been detected, “in order to have quality checks”. Hecho de que nada haya salido de esa plant is, para el, una guaranti más de than the system works. In addition, the Federal Food and Drug Administration (FDA) does not approve the plant approval. The ones he managed were now Holland processes, where production was central, according to Correo.
After detecting the initial error, Emergent said that on April 1, only a single part of the pharmacy component was removed. “The descent of a lot of granular pharmaceuticals, even though it was deceptive, was also used during the manufacture of the vaccine, which is a complex biological process and multiple steps”, said the company, quoted by La Republica.
“Johnson & Johnson assumes liability” for the incident, the company said in a statement on Saturday, claiming that it was “working hard” with the Food and Drug Administration (FDA) to obtain approval living in the Baltimore plant, says Diario Las Américas.
Asimismo, J&J states that “there is a growing body of dedicated to operations and quality, and significantly increases the amount of manufacturing operations staff, quality and techniques to work with the company’s specialists that exist in Emerg.
Coagulants in the song: the Johnson & Johnson vaccine problem
An advisory committee to the CDC will meet today to debate how Johnson & Johnson’s Covid-19 vaccine should be used in the future following reports of rare but serious cases of blood clots. #WSJWhatsNow pic.twitter.com/s93VecUEZM
– The Wall Street Journal (@WSJ) 14 April 2021
In addition, he said that he was waiting for the big sale to require only one dose, his administration was paused by the regulators while reviewing the investigation information on the formation of cerebral palsy in which he reports Reuters.
The cases observed are “extremely rare” for “the safety of the vaccine for the COVID-19 virus is a priority for the federal government”, affirming that the recommendation of a break in the administration should be decided as a precaution. “As soon as the process is complete, we recommend a break in the use of this vacancy by precaution”, said in a statement communicated to the Dra. Anne Schuchat, deputy director of CDC, and dr. Peter Marks, Director of the FDA’s Biological Evaluation and Investigation Center.
Finally, Johnson & Johnson Pharmaceuticals is referring to this issue in the production of its vehicles, signaling that the man will be working with Emergent and the FDA to reach out any way to the final inspection.
Janssen (Johnson & Johnson)’s vacancy was administered to 6.8 million people in the United States when a suspended suspension was made one week to investigate its relationship with unfortunate tornadoes https://t.co/zTudHcborO
– elDiario.es (@eldiarioes) 20 April 2021
“At the moment, there is a special premature about any potential impact that this may have at the time of the entry of our vacancies”, said the company, which has already sent 100 million doses of its vaccine to EE.UU. during the first meeting in 2021. Hasta now Johnson & Johnson has already raised 18 million doses.
#Atento The European Medicines Agency says it should include in the etiquette the secondary effects of the Johnson & Johnson vaccine while finding a possible relationship between biology and the formation of coagulas in adult adults pic.twitter.com/66kl23aCue
– Diario La República (@larepublica_co) 20 April 2021
LEARN MORE: COVID-19: Alaska Vacationing For Tourists From When?