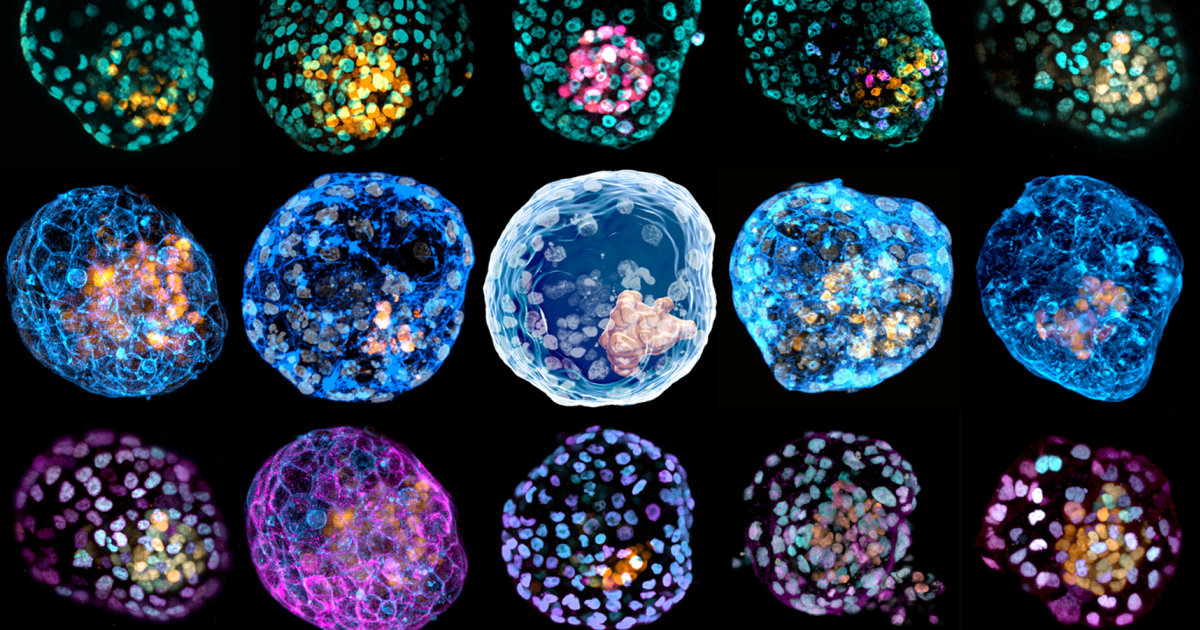
For the first time, scientists have used human cells to create structures that mimic the earliest stages of development, which they say will pave the way for more research without limiting the restrictions on the use of real embryos.
Two articles that appeared in the journal Nature on Wednesday set out how two teams of scientists had made such structures independent.
They stressed that their work is for research only, not for reproduction, but that it is likely to raise new ethical questions.
‘Studying early human development is really difficult. It’s basically a black box, ”said Jun Wu, a stem cell biologist at the University of Texas at Southwestern Medical Center.
“We believe that our model can open up this field,” he said, “if you can test your hypothesis without using human embryos.”
Wu’s team used embryonic stem cells and the second team used reprogrammed skin cells to produce cells that look like one of the earliest stages of human development.
These balls, called blastocysts, form a few days after an egg is fertilized, but before the cells attach to the uterus to become an embryo. To distinguish their models from blastocysts created by fertilization, the researchers refer to the structures as “iBlastoids” and “human blastoids.”
“They should not be considered equivalent to a blastocyst, although they are an excellent model for some aspects of biology,” said Jose Polo, an epigeneticist at Monash University in Australia, who led the second research team. said.
Both groups emphasized that the structures they made were not the same as naturally occurring embryos, and that it was unclear whether they could develop into viable embryos.
“The blastoids are less effective in terms of generating structures that mimic human embryos at a later stage,” Wu said.
Scientists have previously generated similar structures from mouse cells in a laboratory, but this is the first time they have been made from human cells. The new models correspond to about three to ten days after fertilization, Wu said. Last year, researchers unveiled structures that model cells 18 to 21 days after fertilization.
Research involving human embryos and blastocysts is not currently eligible for federal funding in the US, and several states completely ban it.
Some scientists now use blastocysts donated from fertility clinics for research into the causes of infertility and congenital diseases. The new work should enable them to do such research on a much larger scale, Polo said.
“This ability to work on a large scale will revolutionize our understanding of these early stages of human development,” Polo said.
The scientists emphasized that their creations were not meant to be used for human reproduction.
“There is no implant,” says Amander Clark, a stem cell biologist at the University of California, Los Angeles who co-authored the article with Polo. “These structures are not transferred to a uterus or uterine-like structure,” she said. “There is no pregnancy.”
The distinction between blastocysts derived from fertilization and the structures created in a laboratory may not be so clear, said Shoukhrat Mitalipov, a human embryologist at Oregon Health and Science University who was not involved in the research. .
“Both groups show how good it looks like real embryos,” he said. “If they are really as good as embryos, should they be treated as embryos?”
“It brings with it new ethical issues,” he said. ‘Are they going to be covered as human embryos? Should restrictions apply? ”
Scientists had previously tried to convert the laboratory-generated mouse structures into embryos, but to no avail.
The optimal scenario for research is to ‘get as close as possible to a real embryo so that you can learn from it, but not a real embryo, so that you can not enter into debates about the moral status of embryos,’ said Alta Charo, a professor emeritus in law and bioethics at the University of Wisconsin who was not involved in the papers.