For the first time, some Oregonians can get a COVID-19 vaccine at their local Walmart or Bi-Mart.
This is an early example of how the arrival of a third vaccine, made by Johnson & Johnson, could reform the attempt to vaccinate all Oregonians. The new medication requires only one shot and can be stored in a normal refrigerator.
The state used about half of its first grant of the Johnson & Johnson shot, 15,400 doses out of a total of 34,000, to add the two new partners to the pharmacies receiving direct vaccines, along with Safeway-Albertsons, Costco, and Walgreens.
Patrick Allen, director of health care in Oregon, said Walmart needs some time to get its vaccination program up and running, but the shots may be available from the weekend.
Health officials in rural provinces welcomed the news, saying it would improve access to the vaccine for poorer residents who do not have enough time and resources to travel to a mass vaccination site or pop-up clinic.
“Walmarts are often in areas with a lot of poverty, so we are delighted to hear that they will increase access to counties without more prosperous partnerships,” said Sarah Poe, director of the Malheur County Department of Health.
The state expects a delay of two weeks before the next award of Johnson & Johnson vaccine, due to delays in production.

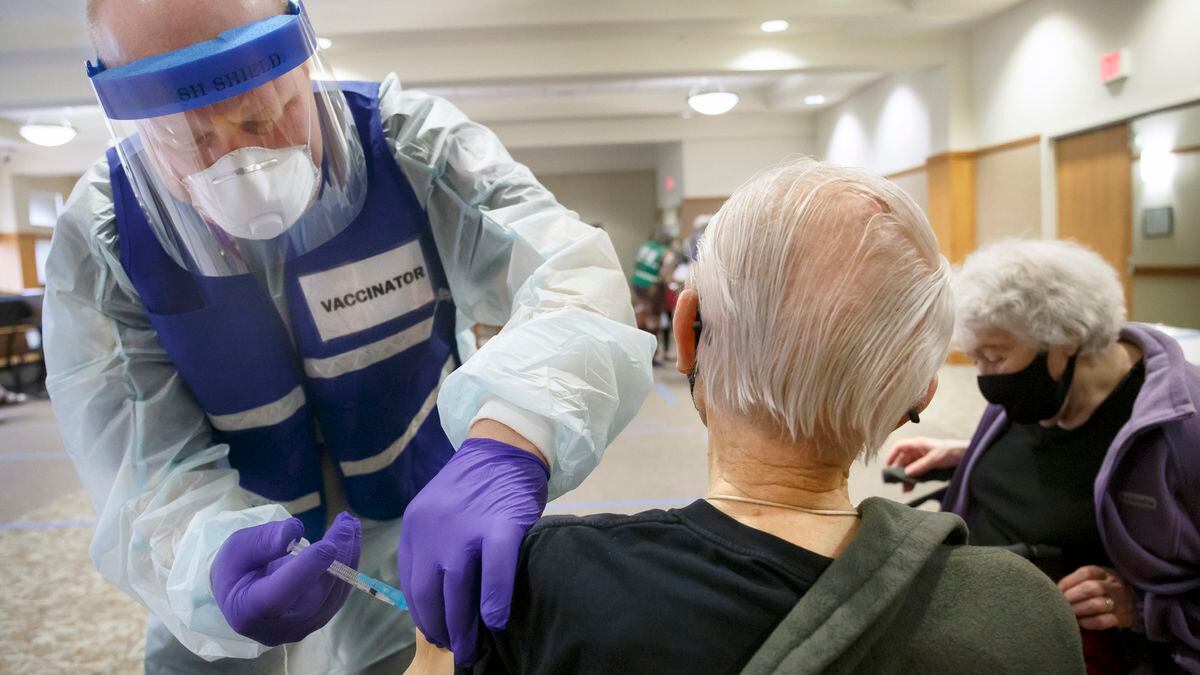
Amity volunteer firefighter and EMT Nic Sherman gives Friendsview Retirement Community resident Sam Farmer a COVID-19 vaccine, as Dorothy Farmer witnessed, in Newberg, Ore., February 5, 2021. As of this week, three COVID-19 vaccines are available in Oregon.
Kristyna Wentz-Graff / OPB
Oregon officials are still developing a strategy for implementing the new vaccine, and state leaders say they can ultimately make decisions at the local level about what population it receives.
OHSU’s mass vaccination center at Portland International Airport received 5,000 doses of Johnson & Johnson version this week, the second largest award.
The rest of the shipment went through the entire state, and each local or provincial public health department received at least 100 doses. Provinces told OPB they plan to use their grants to reach seniors and people in jail.
Four counties – Clackamas, Multnomah, Douglas and Baker – received extra doses that, according to the Oregon Health Authority, are intended to supplement residents in foster homes.
In worldwide clinical trials, the Johnson & Johnson vaccine has been very effective in preventing hospitalization and death. But some health officials, including those in Oregon, are concerned that members of the public may consider the Johnson & Johnson vaccine less effective or less desirable.
“We want to note that we are not creating a perception, but we are giving the less good vaccination to people who are vulnerable and marginalized,” Allen said.
The perception problem fears stem from the challenges of interpreting nuanced clinical trial results.
The Johnson & Johnson vaccine reduces the risk of a participant getting COVID-19 by 66%, and reduces the risk of serious illness by 85%. The Pfizer vaccine was 95% effective in preventing symptomatic COVID-19, while the Moderna vaccine was 91.4% effective.
But scientists say understanding the protection that each vaccine offers against COVID-19 is far more complicated than comparing the percentage efficacy of each trial.
“It’s really too early to distinguish between the three vaccines, except to know that all three protect against serious illness, hospitalization and death,” said Mark Slifka, a professor and vaccine expert at OHSU. “Whatever you can get is the one you have to choose.”
Slifka said several factors make it impossible to make a side-by-side comparison. Each trial took place at a different time, with a different population, versus different versions of COVID-19.
For example, the Johnson & Johnson vaccine was tested worldwide during a high COVID-19 transmission, and the tests included participants in Brazil and South Africa who were exposed to more contagious variants of the virus.
Furthermore, we do not know how long the protection against one of the three vaccines will last, and whether the one can offer more durable protection than the other.
Slifka questions whether the public has already developed a prejudice against the shot. In trials, the Johnson & Johnson vaccine has caused fewer side effects, and as a single dose it is obviously attractive.
“People who do not like needles prefer this one,” he said.