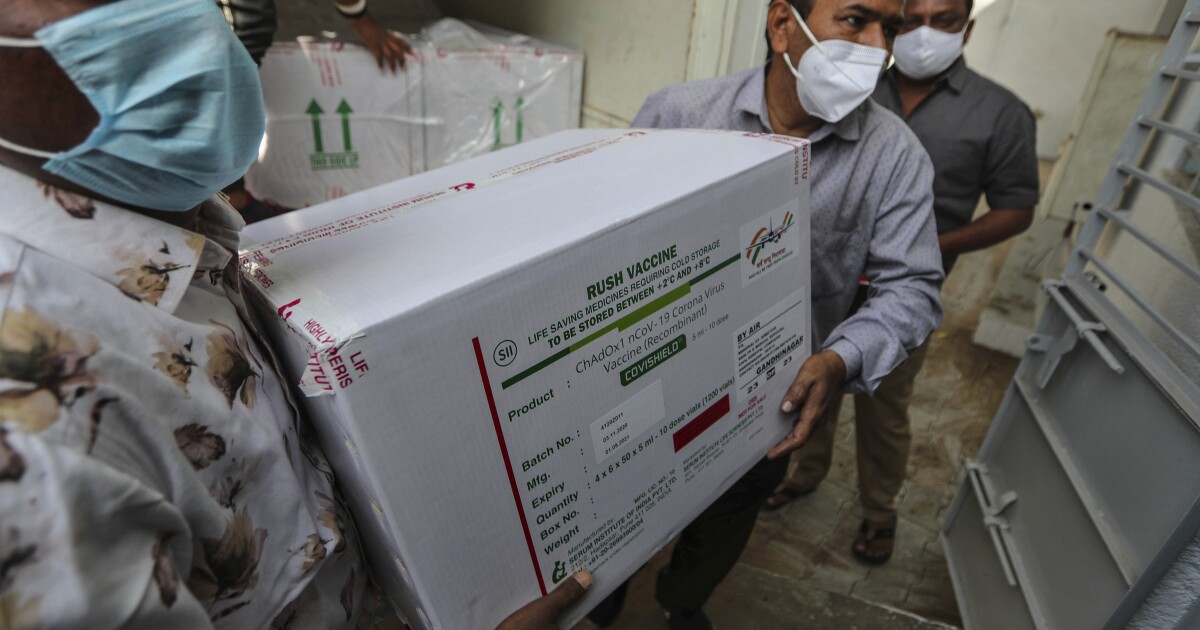
As India makes an ambitious effort to vaccinate 300 million people against the coronavirus within six months, it uses two vaccines – both of which are domestically manufactured but approved under different conditions.
One of these is CoviShield, the vaccine developed by the British AstraZeneca and Oxford University, which according to clinical trials is about 70% effective in preventing COVID-19 and is manufactured in India by the Serum Institute, the country’s largest drug manufacturer.
The other one is Covaxin, developed by an Indian company in collaboration with the government, but the results of which in clinical trials at the late stage have yet to be published. Health authorities have nevertheless approved the vaccine for ‘limited emergency use’.
Government health officials promise that both medicines are effective and say that Indians arriving in the first phase of the shots will not be able to choose which remedy they will receive.
“Many countries around the world use more than one vaccine,” Health Secretary Rajesh Bhushan told reporters on Wednesday. ‘There is no such option [of choice] available to any of the beneficiaries in these countries. ”
Uncertainty over the Covaxin shot is just one challenge facing India as it unfolds one of the biggest vaccinations in world history.

Hindu pilgrims walk past a temporary coronavirus test site in Kolkata, India.
(Bikas Das / Associated Press)
Starting Saturday, vaccines will be administered to 30 million medical professionals and front-line workers, followed by another 270 million people aged 50 and over or those at risk of other diseases. Prime Minister Narendra Modi’s promise to complete the first phase by August will put India, which has the world’s heaviest coronavirus to the United States, on track to defeat COVID-19.
But the populist Modi are known for bold statements that do not always materialize. Experts warn that the lack of transparency surrounding Covaxin could undermine public confidence in the vaccination campaign.
“Whatever happened, it created a perception that the vaccines are not the same,” says Prashant Yadav, a senior fellow at the Center for Global Development in Washington, who studies the Indian health care chain. “It has the potential to cause delays and more friction in a process we ideally want to make as smooth as possible.”
Although China and Russia have also started administering indigenous vaccines while the drugs are still in the trial phase, India is in the process of approving two drugs based on different standards.
The AstraZeneca-Oxford shot was approved by a panel of experts on January 1 based on data from trials in Britain and Brazil. On the same day, the panel asked Bharat Biotech, a well-known manufacturer of more than a dozen vaccines sold worldwide, in Hyderabad to provide more information on the effectiveness of its Covaxin shot, which is still in the critical third phase of the clinical phase was. trials.
The panel approved Covaxin the next day – but publicly gave little explanation as to why. Indian news media also reported irregularities in the testing of the vaccine, and some volunteers said they believe they are getting an approved shot and are not participating in a trial. The company denies allegations of wrongdoing.
The opaque process led to allegations that the government had used the vaccine to promote the nationalist Modi’s mantra of an independent India.
“Controversies always create doubt,” said K. Sujatha Rao, a former Indian health secretary. ‘The government is therefore definitely trying to communicate confidence in the security aspects. But the process can affect perceptions. ”

A health worker examined people in September for symptoms of COVID-19 in Dharavi, one of Asia’s largest slums.
(Rafiq Maqbool / Associated Press)
The stakes are high for a country that has recorded more than 10 million coronavirus infections and 151,000 deaths, one of the most in the world. According to the International Monetary Fund, the Indian economy shrank by 10.3% in 2020, and weeks of protests against new agricultural policies further dampened Modi’s government.
Early fears that the coronavirus would destroy the overcrowded slums and overwhelm rural areas with poor health infrastructure have not yet occurred. India’s daily count of new infections peaked in mid-September and has been steadily declining since then, which officials say is a sign that herd immunity may start to take hold. In the southern state of Tamil Nadu, for example, studies indicate that 60% to 70% of people are exposed to the virus, making them unlikely to be re-infected.
“The number of susceptible people is exhausted or is almost exhausted, so the curve is going down,” said Chandra Mohan, a senior health official in Tamil Nadu. “Under these circumstances, the rollout of vaccines is good, but a little extra time taken here or there to ensure a proper rollout will make no difference.”
To prepare for the vaccinations, India has been holding dress rehearsals nationwide over the past few weeks, with health workers gathering at medical centers, registering patients, rolling up their sleeves and gathering data – all but injecting people.
India relies on an existing vaccination program that routinely administers 55 million people each year. Health officials trained more than 200,000 vaccinators, prepared 90,000 refrigerators and freezers to store the vaccines and rushed them to set up an online portal to locate recipients and drug supplies.
“If all goes well and if the plans are indeed executed according to design, it could very well be an example of how to implement the vaccine on a large scale,” said Yadav of the Center for Global Development.
Among the obstacles is the availability of vaccines. The Serum Institute has pumped out 70 million doses of CoviShield, the AstraZeneca-Oxford shot, and is expected to increase to 100 million doses per month in March, but it will still fall short of the 600 million doses India needs for the first phase. . . The company has indicated that it will focus on delivering India for a few months before exporting doses to other countries.
Bharat Biotech apparently has a stock of 20 million doses of Covaxin, with the aim of producing 700 million more by the end of the year.
Indian officials have indicated that they do not intend to vaccinate all 1.3 billion people in India because they believe the country could achieve herd immunity after the first phase of shootings, affecting less than a quarter of the population will cover.
Experts believe the country has enough health workers, syringes and refrigeration facilities – the vaccines should be kept at a temperature of 35.6 to 46.4 degrees – but are concerned that other vaccinations may be delayed or forgotten as the system focuses on COVID 19.
Anant Phadke, senior adviser to a charity in the western city of Pune, said the infrastructure for vaccinating newborns and pregnant women was already overloaded.
“In the last 40 years, the staff has not increased according to the population,” he said. ‘The existing public health system barely manages the workload. If the existing staff bears a large burden [COVID-19] vaccination, it can make routine vaccinations along the sidelines. ”
Pune, in the state of Maharashtra, is one of the worst affected districts in India, with more than 350,000 coronavirus cases. Officials said their biggest challenge is to reach rural areas, where people regularly have to travel hours to get to a health center.
Bhagwan Pawar, a local health official in the western city of Maan, 100km from Mumbai, said the health centers in the area have enough storage facilities and 48 hours of backup power in case of power outages. Health workers aim to vaccinate 100 people per place per day, he said.
Local health officials declined to comment on the safety of the government-backed Covaxin vaccine. But Avinash Bhondve, former president of the Indian Medical Assn. in Maharashtra, said the medical community has its doubts.
“The government has not ensured that it is 100% safe,” he said.
Times writer Bengali reported from Singapore and special correspondent Parth MN from Mumbai.