La Food and Drug Administration (FDA) de United States alarm to the public about a severe infection of the abdomen with a bacteria which can potentially be risky for health.
Luego de la alerta de la FDA, the company Sunstar Americas Inc. start retiring enjuag GUM Paroex of the market.
The sanitary agency detected that a lot of product is pollution with the bacteria Burkholderia lata, which can only affect persons with debilitating immunological systems or chronic pulmonary diseases, in agreement with the Centers for Disease Control and Prevention (CDC).
Loose CDC exactly what it is bacteria can be resistant to common antibiotics, so you need to seek medical attention to attend a particular case.
La Food and Drug Administration announced a first withdrawal on October 27, this week extending the alert. As of this notice, the agency has registered 29 adverts of people who are positive about the infection por Burkholderia lata.
The use of the paroex buccal juice can provoke oral infections “which requires treatment antibacterial”, Precisó la FDA in a communiqué. In addition, this product can be insured with patients Covid-19.
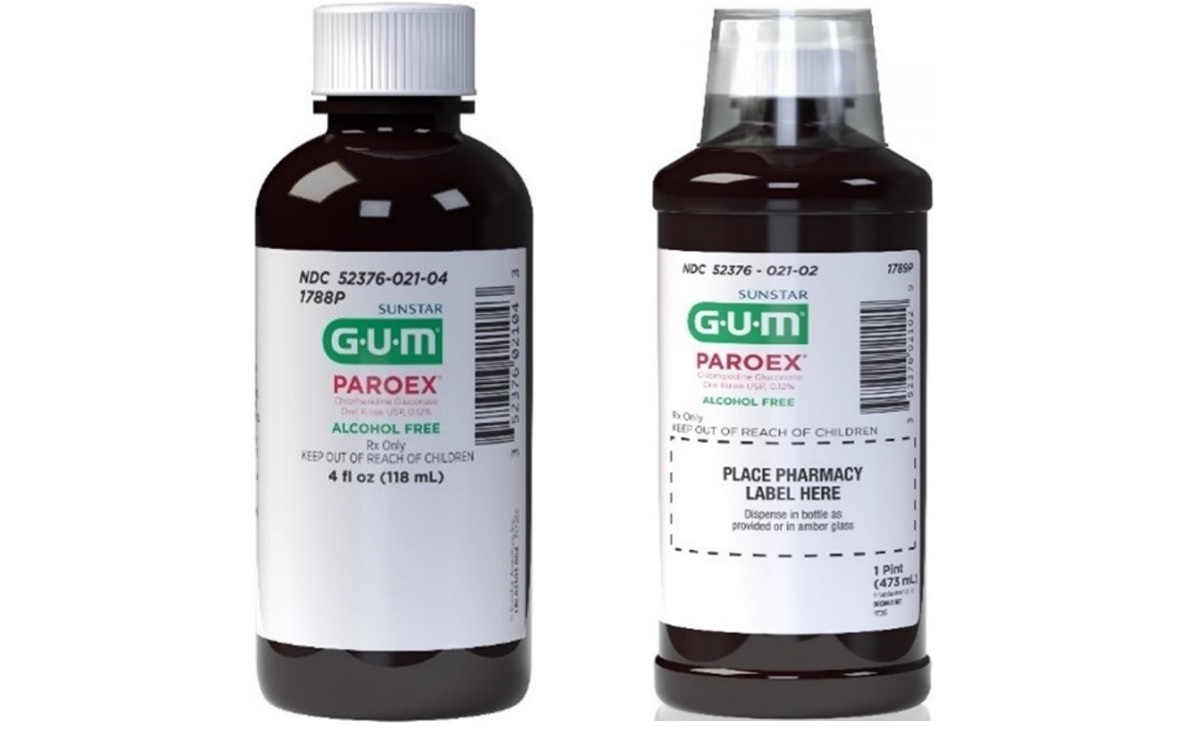
Lots with advertisement for contamination have a gift certificate from 31 December 2020 to 30 September 2022 and will be sold in the following presentations:
– The boxes with six bottles of 16 ounces of GOM Paroex.
– Boxes with 24 bottles of four of us GOM Paroex.
Sunstar Americas Inc. expands voluntary nationwide recall of Paroex Chlorhexidine Gluconate Oral Flush USP, 0.12% due to microbial contamination https://t.co/vQJHkUdoGA pic.twitter.com/OUqRahSUt3
– US FDA recall (@FDArecalls) 29 December 2020
The Sunstar Americas company distributes the enjuag bucal in different regions of the United States for dental consultants, dental distributors, pharmacies, dental schools and pharmaceutical mayors.
The recommendation of the FDA patients and health centers need to use the immediate product and contact a doctor if there are any symptoms of the infection. Burkholderia lata.
bl