They had to do it all holiday. During a pandemic.
“It was a massive effort on the part of many people,” said Scott McAuley, executive director of Piedmont Pharmacy, who had to figure out how to create the Covid-19 monoclonal antibody treatment program.
Studies show that these treatments may prevent Covid-19 patients from developing severe symptoms, yet health officials say not enough of the available treatments have been used.
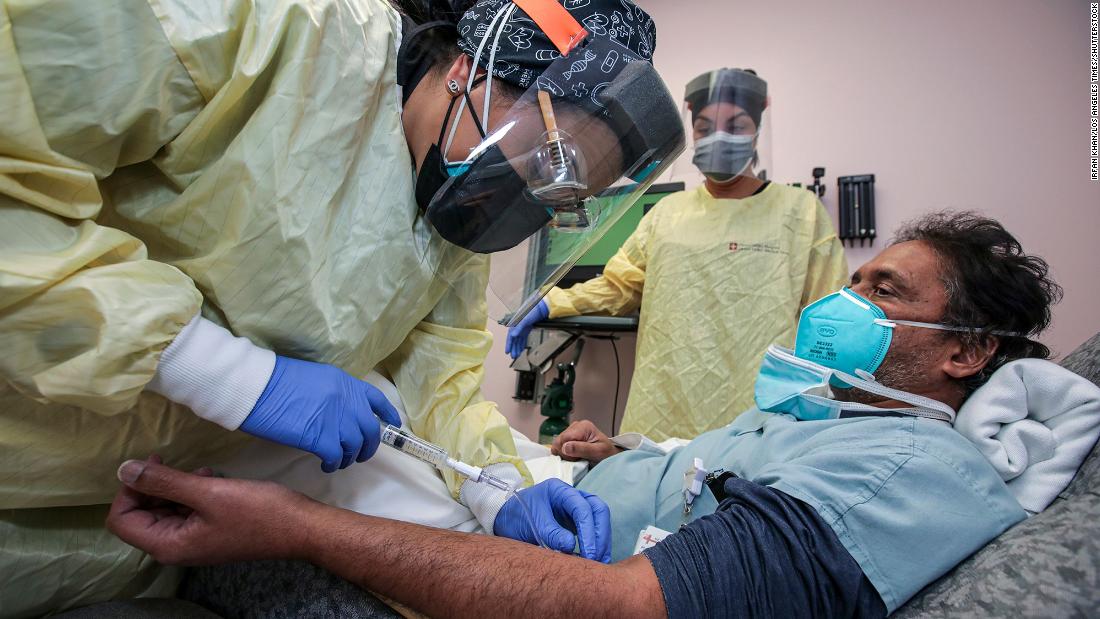
Treating someone is not as simple as swallowing a pill. Because they are contagious, patients undergoing this treatment should be kept separate from others. A nurse in full protective gear should administer the treatment for approximately one hour and then monitor the patient for another.
“It does prevent hospitalizations, but the logistics of it have been frightening. Of course, the staff of nurses in our current national health crisis also have their own struggles,” McAuley said.
The Piedmont program now treats about 250 Covid-19 patients a week, but across the country, health officials said the treatments were not used nearly enough in the months they were available.
In Michigan, for example, less than 10% of the available Covid-19 monoclonal antibody treatments were used, Drs. William Fales, the medical director of the Michigan Department of Health and Human Services, said during a news conference last week.
California health officials told CNN that facilities in their state had 8% of the monoclonal antibody stock on hand during the week of January 13th.
But as the Biden administration launches its own national strategy to control the pandemic, it is not clear exactly how many of the distributed antibody treatments patients actually treated, or where. This information is not posted on the HHS website or retrieved on government panels.
HHS told CNN in early January that the use of antibody therapy was at 25%. Eli Lilly said Wednesday that the use of both antibody therapies approved in the United States has risen to 39%, according to information that Operation Warp Speed shared with the company last week.
“We need you to ask your provider about monoclonal antibodies to keep you out of the hospital,” Adams said.
“The medicine, these therapeutic drugs are not used as much as I, or the doctors of the task force, or the experts, professional experts here in the HHS, feel it should be. I want to remind everyone that we are not helpless in our crusade against the virus. ‘
Aggressive public outreach in progress
An Eli Lilly spokeswoman Molly McCully said in an email that the company was seeing a steady improvement in usage week to week. She added that the company is working with the government to raise awareness about the treatments.
Alexandra Bowie, a spokeswoman for Regeneron, said the company acknowledged that there had been “challenges with the last ten meters in terms of easy administration of the antibody to patients.”
“Our team has worked so hard to develop and test this medicine in record time, and of course we want it to reach as many patients as possible as quickly as possible,” Bowie said in an email.
“As the rollout of antibodies has coincided with an increase in the virus in most states, it is understandably difficult for healthcare providers / centers that are already maximizing,” Bowie said. “But we are making daily calls with government leaders who are responding to patient / physician feedback and working to ensure that more Americans have access to this medicine.”
Regeneron said he also tried to educate providers and make them aware through social media.
Education seems to be working. North Dakota, for example, said it is also trying to make clinicians and the public aware. It is also something that contract trackers call when they reach out to people to let them know they have been exposed to someone with Covid-19.
“Initially, there was a lot of skepticism about this medicine,” said Dr. Joshua Ranum, vice president of the North Dakota Medical Association, said. “Now here has been a very aggressive outreach from the public. I have seen a lot more patient awareness and acceptance and we have seen some dramatic results with efficiency.”
While the use of monoclonal antibody treatments has been ‘low’, it has gradually increased, according to North Dakota Public Information Officer Heather Steffl. According to her, the use of the treatments grew during the first six weeks from 450 infusions to an average of 650 per week.
And it helped. Steffl said the state is successful with the treatments and a decrease in number of hospitalizations.
“There is something they can do to help now”
One healthcare system that has accepted the treatments is Sanford Health in South Dakota, which manages 46 hospitals and 200 senior care facilities in 26 states.
Sanford said it has treated more than 1,400 patients so far with both Lilly and Regeneron antibody treatments.
“Do not get me wrong, there was a bit of construction to do this,” said Dr Jermey Cauwels, Sanford’s chief physician. “But honestly, when we saw that this was something that was going to help us through the worst crisis, we said, ‘Let’s see how quickly we can set this up.’ “
Sanford’s electronic record system was one key to the program’s success, Cauwels said. It is now set to automatically flag when someone tests positive for Covid-19. The system notifies a group that quickly determines if the patient is eligible for treatment, and if so, calls Sanford to get them to the clinic as soon as possible.
Cauwels said the treatments have already prevented at least 40 hospitalizations, multiple deaths and more than a year of hospital days.
“It’s just to do it for a few months,” Cauwels said.
Nurse Dena Ollis, the director of women’s services who helped set up the program in Piedmont Athens Regional, Georgia, said the monoclonal antibody treatments brought something that staff and patients did not see much of during pandemics – hope.
“We were very anxious to set it all up, but once we got it going, it was just very rewarding to see patients so appreciative,” Ollis said. “And the staff, they feel very hopeful that now there is something they can actually do to help the patient and hopefully stop the disease process from getting so bad.”
CNN’s Jacqueline Howard contributed to this report.