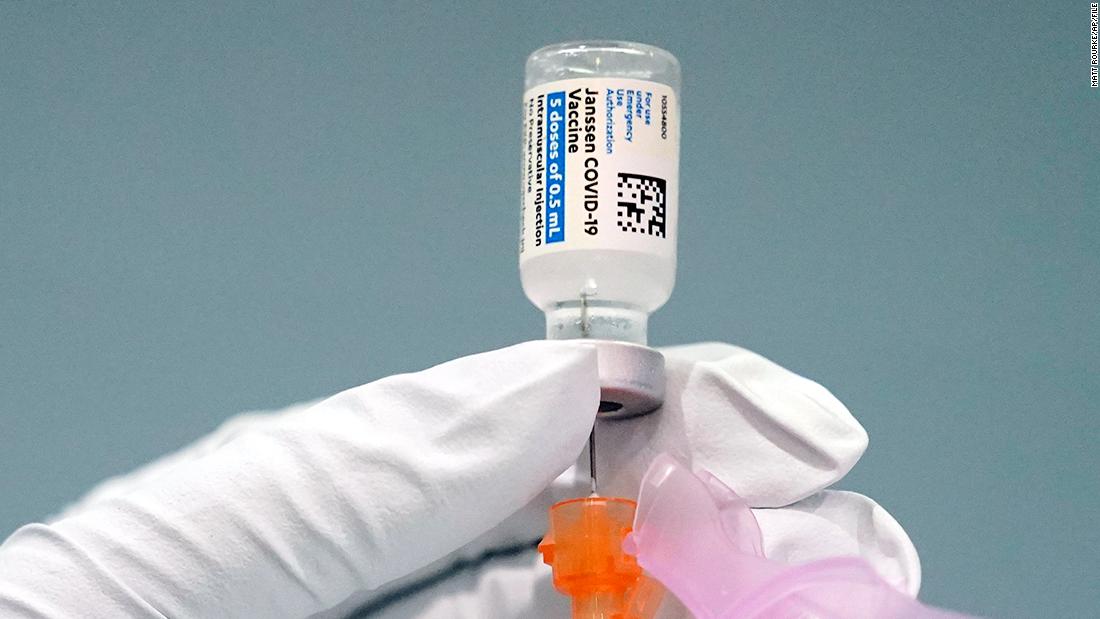
(CNN) – The Centers for the Evacuation of the Centers for the Control and Prevention of Injuries (CDC) will meet these four to make recommendations on the use of the vaccine against Johnson & Johnson’s covid-19 condemn those who are suspended for investigator impossible vinculo con coagulous blood grafts.
The CDC and the Food and Drug Administration of the United States (FDA) recommend a break in the use of the vaccine against J & J’s coronavirus last week in a series of cases reported in the country.
They are investigating if there are more cases and if there are other types of powdery mildew that are associated with the vaccine. The pause also tends to give the intention of the time to the experts to inform the doctors about how to search and treat these coagulants.
The Assessment Committee on the Practice of Immunization (ACIP) of the CDC met on 14 April, but the members said that there was a need for more information about the J&J vaccine and the cases of blood clots.
El Dr. William Schaffner, a member of the ACIP without a vote and a professor of infectious diseases at the Faculty of Medicine of the University of Vanderbilt, told CNN that the committee had taken a decision that probably had more information on coagulation and connection. vacuna, and the members need to understand the demographics of their cases.
Scenarios of what could happen to Johnson & Johnson’s vacancy
Schaffner says that the reunion of the four could unfold different ways.
ACIP can recommend that the use of the vaccine be renewed without change, or the Committee on Recommendation recommends that the use of the J&J vaccine be completed in United States.
Schaffner says it’s most likely that the ACIP will reconsider the use of the vaccine with an ad on possible adverse effects and, potentially, a council on the mayor’s risks to maintain its vacancy completely.
The president of ACIP, the Dr. José Romero, told CNN that the committee also has the option to recommend that the break continue to retrieve more information, even though it has generated enough data on this point for ACIP to make a decision.
Romero dijo que todavía tiene examinating the dates considered the fires, but did not create that the committee decided to recommend a complete interruption of the use of the vacancy in the United States
“The CDC scientists can make an estimate of the series of risk-benefit analyzes, and we will certainly inform you in our decision,” said Romero. “Whoever uses the vacona, as with any vacuna in this country, must be informed about any risk associated with it”.
Romero signaled that it might be necessary to consider a possible dose of refueling of the vaccine against covid-19.
“If there is a high-risk population and if there is any indication that it should not receive the vaccine, I imagine that the CDC will make recommendations on the alternative vaccine series for this group,” said Romero.
What does Johnson & Johnson say?
El Dr. Paul Stoffels, J & J’s scientific director, said on Tuesday that the company would like to see the benefits of the superpowered vacancies.
“The security and benefit of the people who use our product is our number one priority and we firmly support the knowledge and signals of this extremely rare event to ensure correct diagnosis, adequate treatment and detailed information. », Dijo Stoffels.
Romero, who also works as the Arkansas Health Secretary, said he had additional recommendations for the states and physicians on how to administer the J&J vaccine he distributed. Dijo wants the states to accept the committee’s recommendations.
“My advice to the governor is to say that we will advance: ‘We are the ones who say the CDC'”, says Romero. “It’s more likely — that’s more than 98% – to say that it agrees with the committee’s recommendations, and these are the ones we will be debating in our state.”
Although there is a strong concern that the pause of the vacancy J&J will allow the vacancy to be vacated, an inquiry by Axios-Ipsos published on Tuesday showed that 88% of the stadium members think that the CDC and the FDA are acting responsibly the break.
“Really think, and really hope that the public will take a break and see what we have during this break as an indication of what security is the vacancy system and the vacancy process in this country,” said Romero.
Is this the vacancy summit in the United States?
President Joe Biden and other officials have said that any decision on the vacancy against covid-19 will not obstruct the evacuation effort in the United States
The FDA is soliciting the charges against the manufacture of the J&J vaccine in a Baltimore Emergent BioSolutions facility while conducting an investigation into the contamination that affected at least one J&J vaccine lot.
“We will transmit to the general public what we have available vacancies, the Pfizer and Modern, and the people must continue to vacate,” said the CDC Directorate, Dra. Rochelle Walensky.
Walensky said that while the CDC was involved in a risk-benefit analysis of the J&J vaccine, the agency communicated with more than 10,000 recipients to inform about what it needed to do, in case other people experimented with similar ads. .
Those who have received the J&J vaccine in the last three weeks have a very strong desire to unravel the rare coagulation of blood that will decrease with time, say the CDC. The agency recommends that women experiment with certain symptoms, such as cerebral palsy and severity, in addition to the piercings and difficulty breathing, as well as immediate medical treatment.
– Naomi Thomas, Ryan Prior, Jen Christensen, Virginia Langmaid, Ashley Ahn and Jacqueline Howard de CNN make an informal contribution.