A nasal antiviral drug used by researchers at Columbia University Vagelos College of Physicians and Surgeons has the dispatch of EARS-CoV-2 in ferrets, indicating that the nasal spray may also prevent infection in people exposed to the new coronavirus, including recent variants
The compound in the spray – a lipopeptide developed by Matteo Porotto, PhD, and Anne Moscona, MD, professors in the Department of Pediatrics and directors of the Center for Host-Pathogen Interaction – is designed to prevent the new coronavirus invades the host cells.
The antiviral lipopeptide is inexpensive to manufacture, has a long shelf life and does not require refrigeration. These features make it stand out from other antiviral approaches being developed, including many monoclonal antibodies. The new nasal lipopeptide may be ideal for stopping the spread of COVID in the United States and worldwide; the transportable and stable connection may be particularly important in rural, low-income and hard-to-reach populations.
The study was published in the journal Science on February 17, 2020.
Ferrets a model for respiratory diseases
Ferrets are often used in studies of respiratory diseases because the lungs of these animals and humans are alike. Ferrets are very susceptible to infection with SARS-CoV-2, and the virus spreads easily from ferret to ferret.
In this study, conducted in collaboration with Rory de Vries, PhD, and Rik de Swart, PhD, at Erasmus in the Netherlands, 100% of the untreated ferrets were infected by their virus outbreaks, which approach an environment such as sharing a bed . or near living conditions for people.
Porotto and Moscona have previously created similar lipopeptides – small proteins linked to a cholesterol or tocopherol molecule – to prevent infection of cells by other viruses, including measles, parainfluenza and Nipah viruses. These antiviral compounds have been the biggest challenge in human trials, mainly because the infections they prevent are the most common or serious in low-income contexts.
When SARS-CoV-2 emerged, the researchers adapted their designs to the new coronavirus, in collaboration with Christopher Alabi, PhD, at Cornell University. “One lesson we want to emphasize is the importance of applying basic science to the development of treatments for viruses that occur in humans worldwide,” Moscona and Porotto say. ‘The fruits of our previous research have led to the rapid application of the methods to COVID-19. ”
A paper describing a first generation of the compound and its effect in a 3D model of the human lung will appear for the first time on October 20 in the journal mBio. In this human lung model, the compound was able to quench an initial infection, spread the virus into the lungs, and was not toxic to the airway cells at all.
Lipopeptides prevent viruses from infecting cells
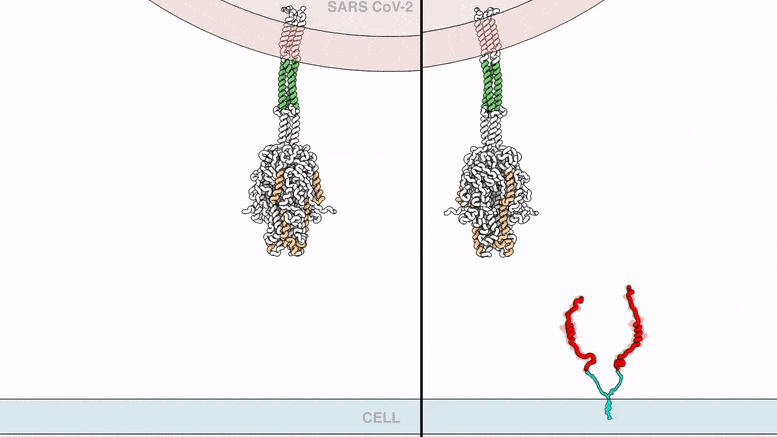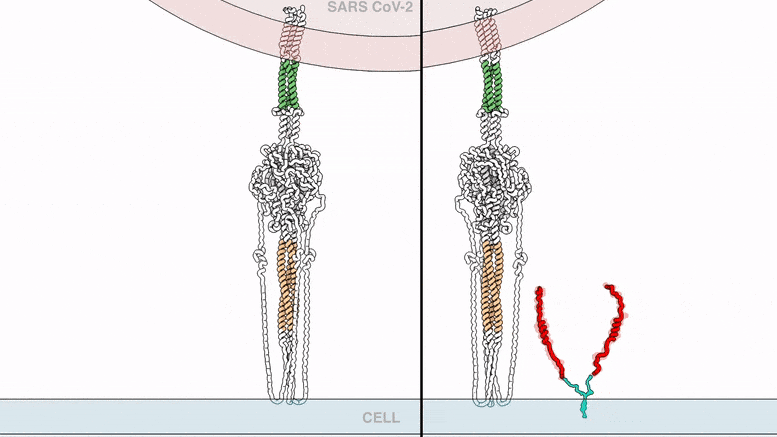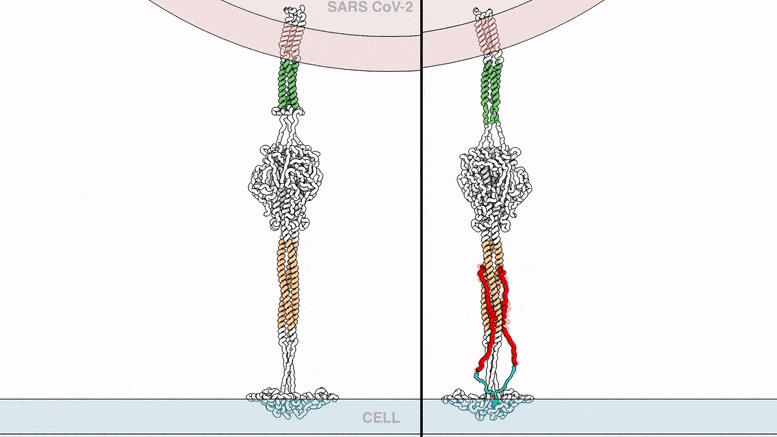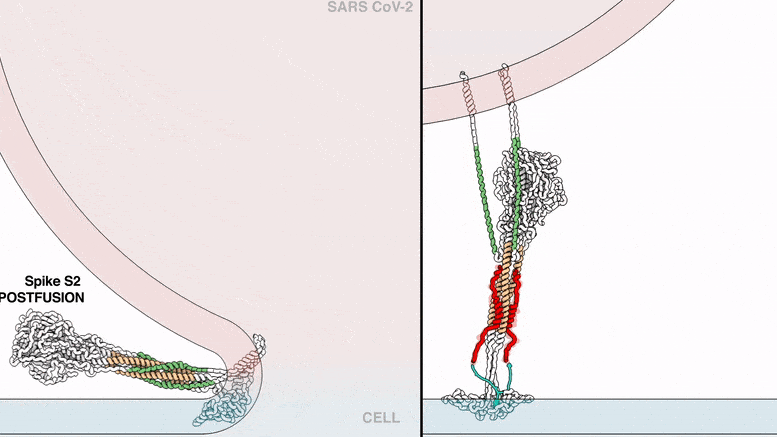
The lipopeptides work by preventing a virus from fusing with the host cell membrane, an essential step that viruses, including SARS-CoV-2, use to infect cells. To fuse, the new coronavirus unfolds its vein protein before contracting into a compact bundle that drives the fusion.
The compound designed by Porotto and Moscona recognizes the SARS-CoV-2 peak, wedge in the unfolded area, and prevents the peak protein from taking the compact form required for fusion.

Anne Moscona and Matteo Porotto. Credit: Photo from the Center for Host-Pathogen Interaction, Columbia University Department of Pediatrics.
In the ferret experiments at Erasmus, the lipopeptide was delivered into the noses of six ferrets. Pairs of treated ferrets were then housed with two control ferrets receiving a saline nasal spray and one ferret infected with SARS-CoV-2.
After 24 hours of direct direct contact between the ferrets, tests revealed that none of the treated ferrets caught the virus from their infected cage mat and that their virus load was exactly zero, while all the control animals were highly infected.
Lipopeptides are effective against variants
Public health officials are concerned about the emergence of several SARS-CoV-2 variants, which appear to be more transmissible and lethal, and may be more capable of evading the antibodies available through current therapies and vaccines.
Porotto and Moscona tested the lipopeptide on cells infected with a series of SARS-CoV-2 variants, including B.1.1.7 and B.1.351, and found that the compound prevents the venous protein of all variants from fusing with the cell membrane as effective as the dominant strain.
Lipopeptides are easily administered
Porotto and Moscona suggest that these peptides can be used in any situation where an uninfected person is exposed, whether in a household, school, health care or a community.
“Even in an ideal scenario with large sections of the population being vaccinated – and with full confidence in and adherence to vaccination procedures – these antiviral drugs will be an important supplement to protect individuals and control the transmission,” says Moscona and Porotto. People who cannot be vaccinated or do not develop immunity will especially benefit from the spray.
The antiviral drug is easily administered, and based on the experience of scientists with other respiratory viruses, the protection will be immediate and last for at least 24 hours.
The scientists are conducting advanced studies on the transfer of animal models and the production and formulation of the peptide. They hope to place this preventative approach in human trials soon, with the ultimate goal of using the therapy to contain transmission during this pandemic and to support preparedness for future emerging strains and pandemics.
References:
“Intranasal fusion inhibitory lipopeptide prevents direct transmission of SARS-CoV-2 in ferrets” by Rory D. de Vries, Katharina S. Schmitz, Francesca T. Bovier, Danny Noack, Bart L. Haagmans, Sudipta Biswas, Barry Rockx, Samuel H Gellman, Christopher A. Alabi, Rik L. de Swart, Anne Moscona and Matteo Porotto, 5 November 2020, bioRxiv.
DOI: 10.1101 / 2020.11.04.361154
“Inhibition of entry into Coronavirus In vitro and Ex Vivo by a lipid-conjugated peptide derived from the SARS-CoV-2 Spike Glycoprotein HRC Domain ”by Victor K. Outlaw, Francesca T. Bovier, Megan C. Mears, Maria N. Cajimat, Yun Zhu, Michelle J. Lin, Amin Addetia, Nicole AP Lieberman, Vikas Peddu, Xuping Xie, Pei-Yong Shi, Alexander L. Greninger, Samuel H. Gellman, Dennis A. Bente, Anne Moscona, Matteo Porotto, 20 October 2020, +mBio.
DOI: 10.1128 / mBio.01935-20
Anne Moscona, MD, is the Sherie L. Morrison Professor of Immunology (in Microbiology and Immunology), Professor of Pediatrics, and Professor of Physiology and Cellular Biophysics at Columbia University Vagelos College of Physicians and Surgeons.
Matteo Porotto, PhD, is an associate professor of viral molecular pathogenesis in the Department of Pediatrics at Columbia University Vagelos College of Physicians and Surgeons.
Other authors: Rory D. de Vries (Erasmus University Medical Center, The Netherlands), Katharina S. Schmitz (Erasmus), Francesca T. Bovier (Columbia University Irving Medical Center and University of Campania “Luigi Vanvitelli”, Italy), Danny Noack ( Erasmus), Bart L. Haagmans (Erasmus), Sudipta Biswas (Cornell University), Barry Rockx (Erasmus), Samuel H. Gellman (University of Wisconsin, Madison), Christopher A. Alabi (Cornell), and Rik L. de Swart (Erasmus).
This work was supported by funding from the National Institutes of Health (AI146980, AI121349, NS091263 and AI114736), the Sharon Golub Fund at Columbia University Irving Medical Center, the Children’s Health Innovation Nucleation Fund of the Department of Pediatrics at CUIMC, and ‘ a COVID -19 Research Award from Harrington Discovery Institute at University Hospitals.
Anne Moscona, Matteo Porotto, Rory de Vries, Francesca Bovier and Rik de Swart are listed as inventors in a preliminary patent application covering findings in this article.