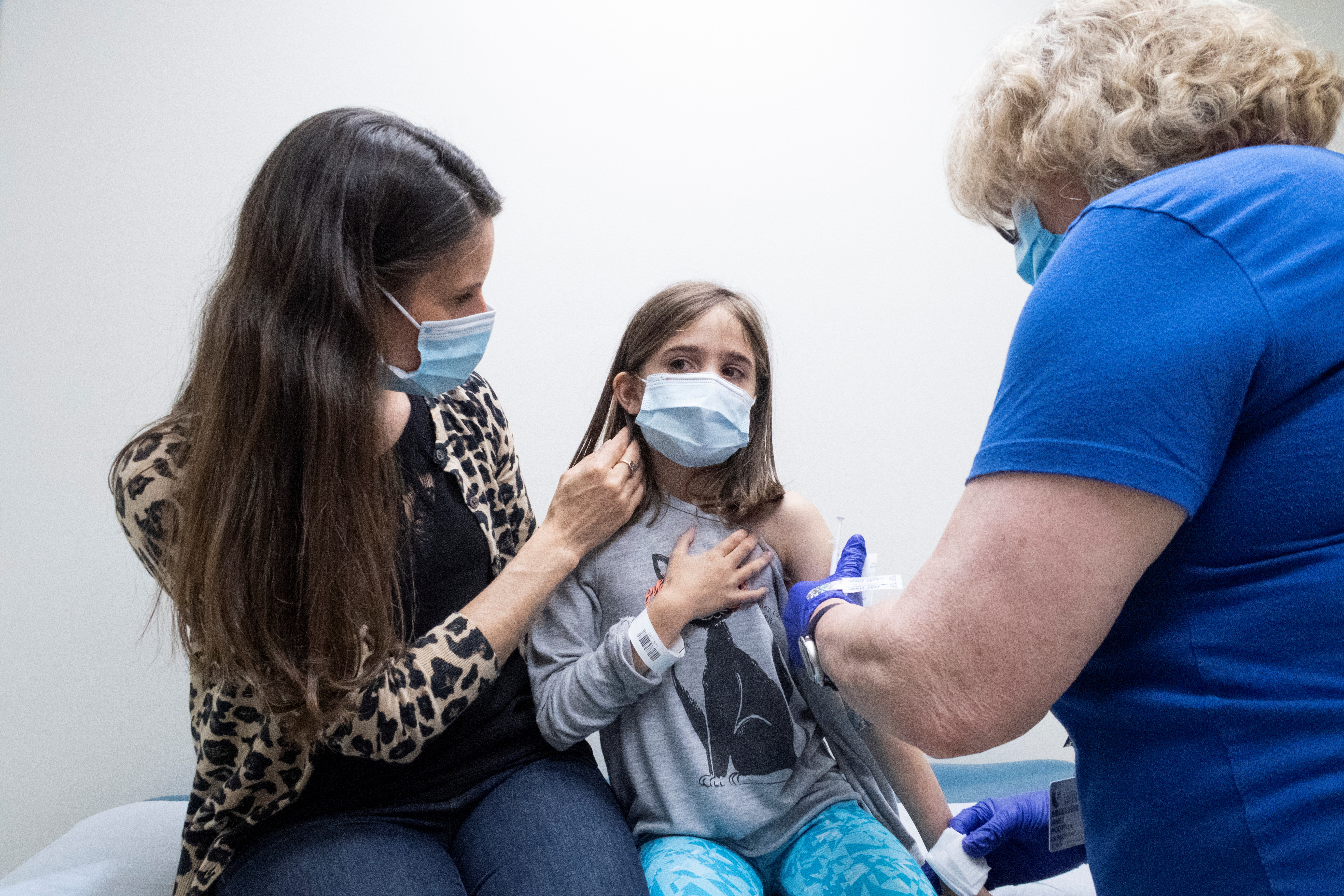
Tristen Sweeten, a 34-year-old nurse in Utah, hopes her three children will receive Moderna (MRNA.O)’s COVID-19 vaccine through his clinical trial for children. The sooner the better, she said for their safety and the greater the goal to end the pandemic.
Angie Ankoma, a 45-year-old black mother of four children working in philanthropy in Rhode Island, believes trials should have included different populations and participated in one for a COVID-19 vaccine. Volunteering her children for possible inclusion in the trial of Moderna was a more difficult call.
Sweeten and Ankoma are among thousands of American parents who voluntarily involve their children in new trials conducted by Pfizer (PFE.N) with BioNTech or Moderna, the first companies to develop a safe COVID-19 vaccine for the 48 million land. children under 12 years.
Health officials say vaccines are essential to end the pandemic. But many are worried about vaccinating vaccines in some adults, it will be even more obvious when it comes to their children. Parents may question the risks to the benefits, given the unknowns about the long-term impact of the vaccines on the development of children and the data on how few young children were severely affected by COVID-19.
To alleviate the problems, some scientists say the US Food and Drug Administration should slow down the review process for COVID-19 vaccines for children.
Pfizer spokeswoman Jerica Pitts said it was premature to speculate on a child approval route, but the company plans to work with public health agencies to promote the importance of vaccines.
Dr. Jacqueline Miller, researcher at Moderna, said the company spoke to the FDA about the best way to clean the vaccine for use in children. She said the company hopes to make the vaccine available to children through emergency use authorization (EUA), which has brought it to American adults in record time, in part to get children back to school “and the things they all do taste. do. ‘
Sweeten’s husband, Scott, is a clinical researcher whose company worked on the Johnson & Johnson (JNJ.N) and AstraZeneca (AZN.L) adult vaccination tests. The couple, whose children are between the ages of 5, 8 and 10, are comfortable with how they were developed, Tristen said.
“We feel like they’s very safe,” she said.
Ankoma consulted her pediatrician because she had doubts about unknown long-term consequences. Eventually, she decided that the risk was worth immunizing her four children, between the ages of 7 and 16.
“It was easier for me to decide for myself than for the kids, because … it was my own body,” she said.
‘THAT GOLDILOCKS MOMENT’
Researchers giving rise to pediatric trials for Moderna and Pfizer in children as young as six months are confident that the vaccinations will be just as safe and effective for children as for adults.
Pfizer’s vaccine, which is already available to people aged 16 and over in most US states, has been found to work well in children aged 12 to 15 and may receive regulatory authorization for that age group next month.
Moderna and Pfizer said vaccines may be widely available to even younger children in early 2022.
A poll by Axios / Ipsos from April 2 to 5 found that only 52% of American parents said their children were likely to be vaccinated as soon as they were eligible.
Children under 12 have so far had a relatively low risk due to the coronavirus.
About 284 children have died from COVID-19 since last May, about 0.06% of all COVID-19 deaths, according to the American Academy of Pediatrics in about 43 states. There were 14,500 hospitalizations among children in 24 states during that time, about 2% of the total.
Dr. Sean O’Leary, a professor of pediatrics at the University of Colorado, said vaccination will help children avoid hospitalizations, a rare inflammatory reaction or persistent symptoms known as long COVID.
‘It is certainly not correct to say that it is benign in children. “Anyone who has worked in a children’s hospital can tell you how many sick children we have cared for,” he said.
Children are already receiving vaccines for diseases with similar or lower levels of related mortality in children, such as hepatitis A, varicella, rubella and rotavirus.
Health officials warn that children, if not vaccinated, can be a reservoir for infections, which can lead to virus variants that can spread and grow.
The confidence in parents should be that these vaccines will be commonly used in adults before they are made available to children, said Emmanuel Walter, head of Pfizer’s childhood vaccine trial at Duke University.
Some other vaccines have been developed for and given only to children, such as chickenpox.
More than 63 million Americans received the Pfizer vaccine and about 55 million received the Moderna shot.
The trials for young children are more involved than for adolescents, as they start by testing very small doses and gradually increase the dose while looking at side effects.
“What we are trying to find is that the Goldilocks moment is that we have just enough vaccine to generate a very good immune response, but we do not have so much that we cause a lot of fever and pain in the arm. Baby or in the younger child, Says Buddy Creech, a professor at Vanderbilt University who is working on the pediatric study of Moderna.
Some scientists said that waiting for standard approval instead of seeking an EUA would add months to the roster, but that it would be possible to gather more safety data that could boost public confidence.
The FDA declined to comment.
Dr. Cody Meissner, head of pediatric infectious diseases at Tufts University’s medical school, said it came down to one question: “Does the low disease burden in children justify a longer-term evaluation of safety?”
Our standards: the principles of the Thomson Reuters Trust.