In the large variety of microbial lives, there are numerous examples of symbiosis – a process by which different organisms maintain a stable relationship with each other. Such interactions usually provide some benefit to one or more involved organisms. Subscribe Nature, Graf et al.1 reports the discovery of an interesting example of symbiosis between microorganisms. This finding may shed light on the types of processes that led to the evolution of mitochondria, the energy-producing organelles found in eukaryotic cells (those that contain a nucleus).
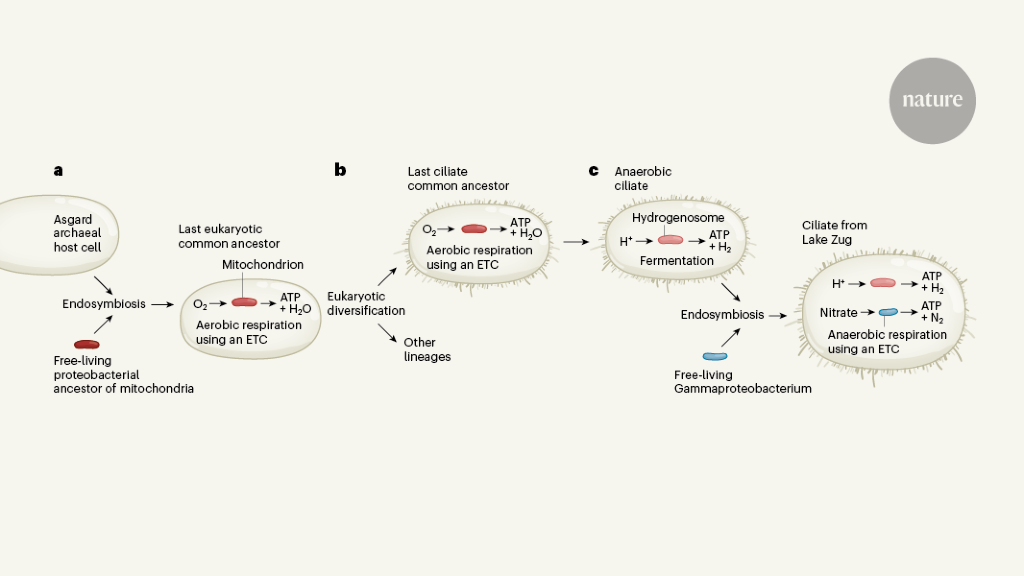
In some cases, symbiotic partners can be integrated in such a way that one partner is taken up into the cell by the other partner through a process called endosymbiosis (Fig. 1). Endosymbiotic interactions have enabled important transitions throughout the history of life on earth. A good example of this is considered to be the interaction that gave rise to the first eukaryotic cell. These ancestral eukaryotes probably formed from an endosymbiosis in which a host cell belonging to a group of unicellular organisms called Asgard archaea2,3 took up a type of bacterial cell that belonged to the phylum Proteobacteria4. Mitochondria in eukaryotic cells are considered to be the direct descendants of these once-free proteobacterial endosimbions.
Grave and colleagues describe an interesting bacterial endosymbiont they found in a silicate, a type of eukaryotic, unicellular microbe. Although associations between these types of organisms are not uncommon, this case has some features of the type of endosymbiosis that gave rise to mitochondria. This includes the production of energy in the form of ATP molecules through the energy generation process known as respiration. This process is also linked to a mechanism by which ATP can be carried out from the endosymbiont to supply the host with energy.
The environmental conditions and atmospheric composition of today differ markedly from the conditions that existed when life first appeared on this planet more than four billion years ago. During the launch of the Great Oxidation Event, about 2.1 billion years ago5, oxygen that accumulated in the atmosphere and ocean waters, a development that had a major impact on life on earth. Oxygen is toxic to most organisms that thrive in environments without environments. However, some microorganisms have learned to utilize the chemical properties of oxygen by using it as an electron acceptor in energy generation pathways. This type of oxygen-dependent process, called aerobic respiration, is much more energy efficient than fermentation, a form of non-oxygen-dependent energy metabolism of ancient origin, which enables many anaerobic organisms to survive without oxygen.
Eukaryotic cells probably originated somewhere after the Great Oxidation Event6, and their ability to perform aerobic respiration was acquired by the mitochondrial endosymbiont. The ability to provide efficient energy production is thought to have selective benefits during eukaryotic evolution, although the exact contribution to, for example, the emergence of cellular complexity is discussed.7.
Current evidence6 indicates that early eukaryotic evolution and diversification took place under conditions in which oxygen was present. However, some groups of eukaryotes still thrive in oxygen-free environments. These anaerobic eukaryotes are thought to be developed from aerobic mitochondrion-bearing ancestors.
In the absence of oxygen, the anaerobic eukaryotes use a fermentation-based metabolism, which subjects them to stricter energy regimens than those of aerobic eukaryotes. This type of metabolism originated in different types of anaerobic eukaryotes, and is associated with the development of mitochondria in organelles called hydrogen fosomes.8. All hydrogen fosomes have, to some extent, lost mitochondrial pathways for aerobic respiration, generating hydrogen rather than carbon dioxide and water as an end product of their energy-generating pathways. Ciliates are extremely effective at adapting to oxygen-depleted environments, and mitochondrion-to-hydrogenosome transitions have occurred several times independently in this group of organisms.9.
Grave and colleagues investigated an anaerobic silage of the class Plagiopylea, found in the deepest layers of Lake Zug in Switzerland. This environment has no oxygen and contains relatively high levels of nitrate. An initial assessment using microscopy revealed that these ciliates, unusually, are a bacterial endosymbiont (belonging to the class of Gamma proteobacteria), rather than an archaeal endosymbiont that produces methane, which is the more typical type of endosymbiont is found in anaerobic ciliates.
DNA sequencing for more water samples showed the presence of genes indicating that the ciliary cells have hydrogen fosomes. In addition, the sequential data indicate that the bacterial endosymbionts have an electron transport chain – a collection of protein complexes for respiration that can produce energy through a process called oxidative phosphorylation. Grave et al. suggests that the electron acceptor in this chain is nitrate, rather than the oxygen used by aerobic organisms. Consistent with this model, the authors report that the rate of denitrification (the microbial process that converts nitrate to nitrogen) was higher in lake water samples in which ciliates were present than in those where ciliates were removed.
The genome of the endosymbiont identified by Graf and colleagues is remarkably smaller than the genome of most endosymbionts of microbial eukaryotes, which contains only 310 protein-coding genes. Among those, the authors identified a gene that encodes a potential transport protein for ATP, which they say is used to export ATP from the endosymbiont into the host, enabling the ciliate to use the endosymbiont for energy production through nitrate ‘to breathe’. This finding is a unique example of an endosymbiont that contributed the ability to breathe (although nitrate instead of oxygen as an electron acceptor) to a eukaryote that apparently retains mitochondrial-derived organelles (hydrogen fosomes) – the ancestral versions of which were once respiratory. was functions.
Strikingly, several parallels can be drawn between the cellular partnership discovered by Graf and collaborators and the evolution of mitochondria in eukaryotes. In both cases, respiratory capacity is gained by an anaerobic host cell through the metabolic integration of a proteobacterial endosymbiont, and mechanisms can be identified for the exchange of energy between symbiont and host cell. Furthermore, a significant reduction of the endosymbiont genome is observed, although mitochondrial genomes are typically much smaller than the endosymbiont genome observed by Graf and colleagues, or have been completely lost (as is the case with several hydrogenosomes).10).
Despite these fascinating similarities, there are also striking differences. Mitochondrial endosymbiosis was a very ancient event, employing an archaic host cell rather than a modern eukaryotic cell. Mitochondria, even if they are now reduced from their original form, or even lost by some contemporary eukaryotes11, has become an integral part of eukaryotic cells. Genes inherited from the original mitochondrial endosymbiont are often re-targeted to the nuclear genome, and some of the proteins encoding these genes have assumed different functions throughout the cell. A similar level of integration is unlikely for the bacterial endosimbions of the ciliate examined.
It would nevertheless be interesting to investigate whether genes have been shifted or reused between the host and endosymbiont, and to what extent a typical mitochondrial function, such as ATP production, has been replaced or retained in the hydrogen of the cilia . Evidence indicating that the ATP transporter identified by Graf and colleagues could export ATP to the ciliate host would help confirm the proposed symbiotic interaction. Further discovery and exploration of similarly surprisingly symbiotic interactions in parts of the microbial world that are poorly explored is certainly an exciting prospect for the future.